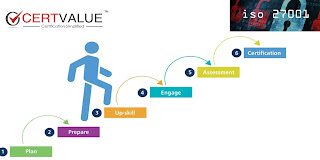
What are the certification steps and Benefits of ISO 13485 Certification?

ISO 13485 Registration in Bangalore specifies requirements for a quality management system wherever a corporation has to demonstrate its ability to supply medical devices and connected services that consistently meet client and restrictive needs. Such organizations are often concerned in one or additional stages of the life cycle, together with style and development, production, storage and distribution, installation, or servicing of a medical device and style and development, or provision of associated activities like technical support. ISO 13485:2016 can even be used by suppliers or external parties that provide products, together with quality management system-related services to such organizations. Certification Steps: Planning the quality system: ISO 13485 includes a quality planning demand. Writing a top-quality manual isn't sufficient, you need documented quality plans for implementing changes to your quality management system. there's no required format for quality...